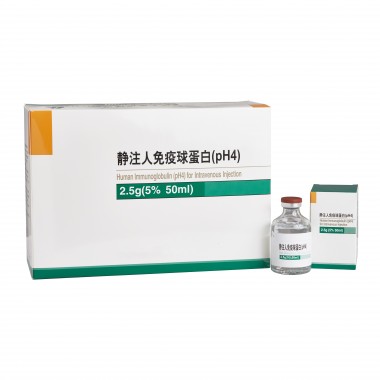
Human Immunoglobulin for Intravenous Injection (pH4)
- FOB Price:Get Latest Price >
- Min.Order:1000 Piece(s)
- Payment Terms:L/C , T/T
- Favorite
Product Information
- Indication:immunoglobulin deficiency
- Specification:10g(5% 200ml), 5g(5% 100ml), 2.5g(5% 50ml), 1.25g(5% 25ml), 1g(5% 20ml)
- Dosage Form:Injection
- Route of Administration:Intravenous Drip
- Shelf Life:2 years
- Storage:2℃-8℃
- Place of Origin:China
- Qualification:Chinese GMP
Description
Target Countries:
Central & South America, Southeast Asia
Highlights
1. Intravenous injection, anti infection & immunomodulatory;
2. Prove to be effective in the COVID therapy;
3. Derived from human plasma with few adverse effects;
4. Complete IgG subclass, conforming to normal human plasma ratio;
5. Wider spectrum of antibodies.
Indications
1. Primary immunoglobulin deficiency diseases, such as X-linked hypoimmunoglobinemia, common variable immunodeficiency disease (CVID), IgG subclass deficiency, etc.
2. Secondary immunoglobulin deficiency diseases, such as severe infections, neonatal sepsis, etc.
Autoimmune diseases (AID), such as primary thrombocytopenic purpura, Kawasaki disease (KD), etc.
Dosage Form
It is a clear, colorless or light yellow liquid. Opalescence may occur but without turbidity.
Specifications
10g(5% 200ml), 5g(5% 100ml), 2.5g(5% 50ml), 1.25g(5% 25ml), 1g(5% 20ml)
Shelf life
24 months
Specific Requirements
Deal in the related industry;
Familiar with local regulation for drug entry;
Familiar with local sales channel and market;
Active and confident in promoting the product.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank