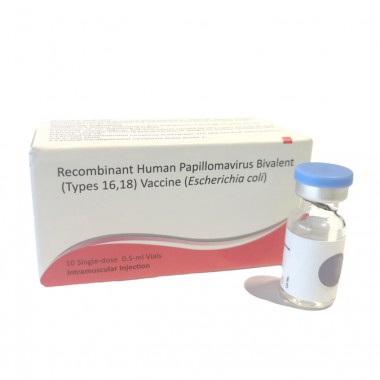
Recombinant Human Papillomavirus Bivalent (Types 16, 18) Vaccine (Escherichia coli)
- FOB Price:Get Latest Price >
- Min.Order:100000 Vial(s)
- Payment Terms:L/C , T/T
- Favorite
Product Information
- Dosage Form:Injection
- Route of Administration:Intramuscular Injection
- Shelf Life:3 years
- Storage:2℃-8℃
- Qualification:WHO PQ
Description
Target Countries:
Central& South America, Southeast Asia
Highlights:
1. Exclusive vaccine to prevent the most common female malignant tumor - Cervical Cancer;
2. Prevent 84.5% of cervical cancer;
3. Target for female aged 9-46 yrs;
4. WHO PQ approved;
5. More affordable price than 4-valent/9-valent HPV vaccine.
Expression System:
Escherichia coli
Formulation:
With Alum adjuvant. No preservative
Target population:
Women ages 9-45years
Schedule:
0, 1, 6 m (0, 6m for 9 -14 yrs)
Shelf-life:
2-8℃ 36 months in application
Specific Requirements:
Deal in the related industry;
Familiar with local regulation for drug entry;
Familiar with local sales channel and market;
Active and confident in promoting the product.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank